Background and Rationale
Honey bee plagues. Honey bees are plagued by a variety of infectious diseases correlated with reduced tolerance to stressors and potential colony collapse. These include viral, bacteria, fungal, and protozoal parasites and pathogens that are linked to overwinter colony collapse, which can exceed 30% per year. Infections can also reach high prevalence in other managed and wild bees that are likewise important for agriculture. A better understanding of these infectious diseases, and ways to augment resistance to such infections, would contribute to preservation of bee populations and cost-effectiveness of their pollination services.
Temperature and disease resistance. One powerful determinant of infection is temperature. Increases in host temperature—also known as fever—have been shown to reduce infection in plants, fish, mammals, amphibians, and insects 12–16. Both endothermic (‘warm-blooded’) and ectothermic (‘cold-blooded) animals can raise their body temperatures when infected, sometimes with disease-curing effects. In bees, particularly social bees like honey bees that can maintain consistently high temperatures (33-37 °C) in their colonies, temperature regulation can deter establishment by less heat-tolerant parasites.
Microbiota and disease resistance. Another factor that can influence infection is the presence of non-pathogenic microbes called symbionts. The symbionts of the gut interact directly with gut parasites and can inhibit parasite growth. Honey bees have a well-characterized gut symbiont community with demonstrated benefits against infection. This community is rich in species of Lactobacillus, the same bacterial genus that is used for food preservation (as in yogurt and sauerkraut) and touted for benefits to human and livestock health.
Interactive effects of temperature and microbiota on infection. Despite the recognized importance of temperature and microbial symbionts in resistance to infection, few studies have explored the effects of temperature on gut symbionts, and the consequences of these effects for disease resistance remain virtually untested. In honey bees, the dominant gut symbionts are generally grown at relatively high (34-37 °C) temperatures in vitro, whereas widespread trypanosomatid and microsporidian gut parasites (Crithidia, Lotmaria, and Nosema spp.) have recommended incubation temperatures of 25-27°C. These differences suggest that high temperatures, such as the 34-36 °C at the core of bee colonies, could favor core gut symbionts over parasites due to the relatively high growth rates of symbionts at these temperatures, and thereby reinforce host-bacterial mutualisms in honey bees. However, the temperature dependence of honey bee parasite and symbiont growth and symbiont-driven parasite inhibition remain an open question.
Aims and Objectives
In this project, we addressed how temperature can alter the interactions between honey bee gut parasites and Lactobacillus gut symbionts. We focused on two protozoal parasites—the trypanosomatids Crithidia mellificae and Lotmaria passim—recently described by former Bee Research Lab scientist Ryan Schwarz. These emerging species have shown over 90% prevalence in some colony surveys and have been associated with colony losses on multiple continents, indicating a need to better understand their biology and factors that might mitigate bee infection. Our previous showed that growth of the related bumble bee parasite Crithidia bombi can be inhibited by both high temperatures and Lactobacillus gut bacteria, suggesting that these same factors could likewise reduce proliferation of honey bee parasites. To evaluate the joint effects of temperature and gut symbionts on these widespread yet understudied parasites, we compared responses of growth to temperature between the two trypanosomatids parasites and Lactobacillus gut symbionts; examined the temperature and chemical dependence of parasite-symbiont interactions using cell cultures; and have begun to test the joint effects of temperature and gut bacteria on bee infection.
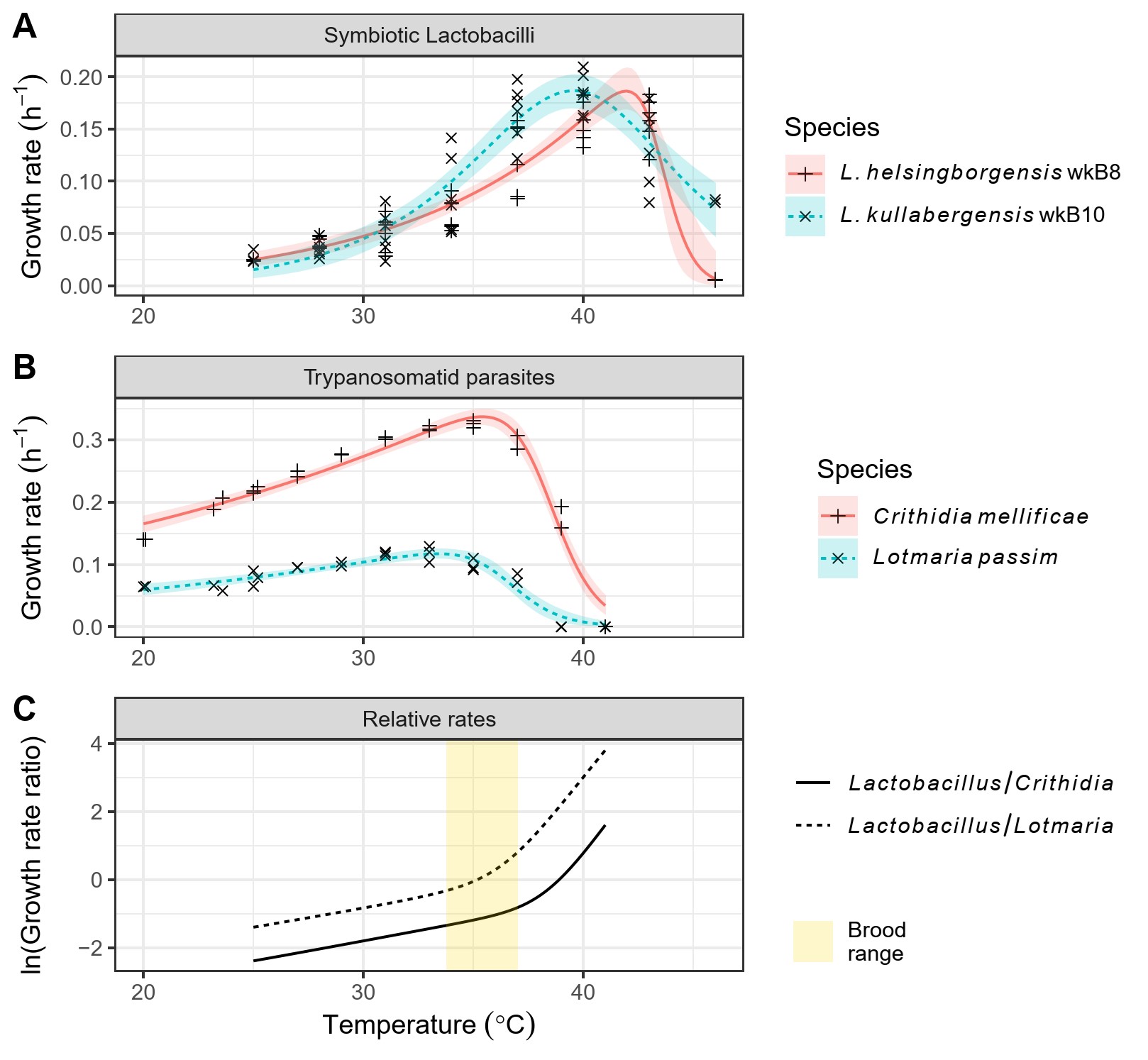
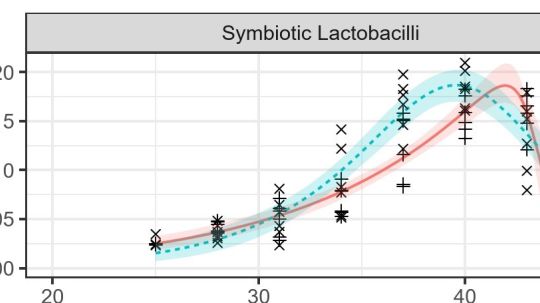
Figure 1. High temperatures favored the growth of Lactobacillus gut symbionts relative to that of Crithidia and Lotmaria parasites.
Outcomes
Temperature-dependent, pH-mediated interactions between bee parasites and symbionts
Our first step was to compare the temperature dependence of parasite and symbiont growth rates. To do this, we measured the growth rates of parasite and symbiont cell cultures over the range of temperatures found in bee colonies, then used non-linear models of enzyme kinetics to compare the temperatures of peak growth and limits of heat tolerance between Lactobacillus symbionts and Crithidia and Lotmaria parasites. We found that high temperatures provided a relative advantage to the (non-pathogenic) Lactobacillus, potentially allowing these bacteria to competitively exclude parasitic trypanosomatids from the host intestine (Figure 1). The relative growth rates of Lactobacillus and Lotmaria changed by over 3-fold over the 4 °C range over which honey bees incubate larvae and pupae at the center of the colony, indicating that small shifts in colony temperature regulation could have significant consequences for the relative abilities of parasites and symbionts to establish in the guts of young worker bees.
In conjunction with these studies of isolated parasite and symbiont cultures, we investigated the potential for chemically mediated parasite-symbiont interactions, and how these change across the range of temperatures found in honey bee colonies. We found that symbionts chemically inhibited parasite growth via production of acids, with inhibition occurring within the pH range previously measured in bee intestines. Acceleration of symbiont growth and acid production at high temperatures resulted in progressively stronger antiparasitic effects with increasing temperature (Figure 2), with symbionts causing >50% reductions in parasite growth rate above 33 °C—very close to the temperatures found at the colony core.
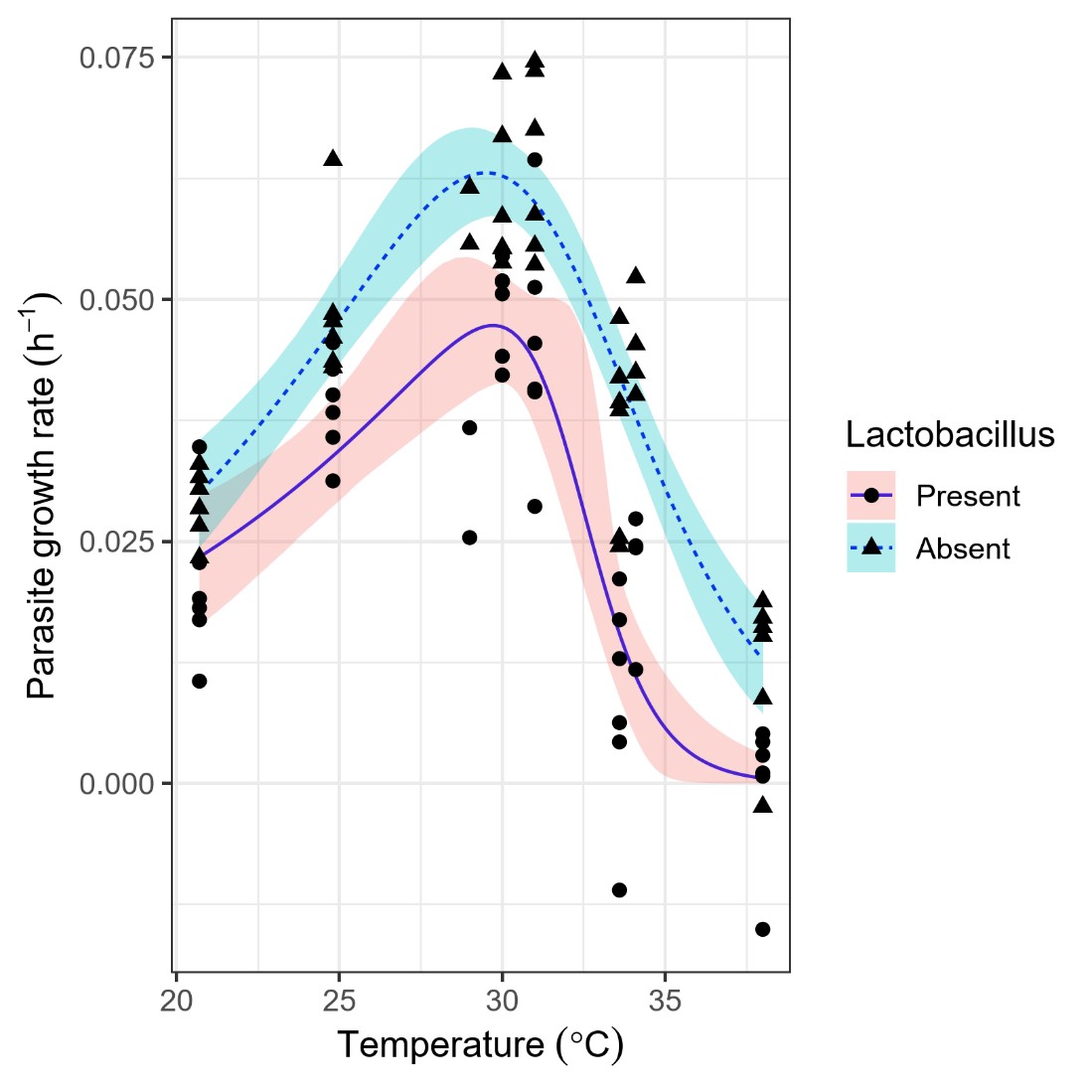

Figure 2. High temperatures increased inhibition of the parasite Lotmaria passim by Lactobacillus gut symbionts.
Conclusions. Our findings suggest that fluctuations in colony and body temperatures across life stages and seasons could alter the protective value of bee gut microbiota against parasites, and that temperature-driven changes in gut microbiota could be an underappreciated mechanism by which temperature— including endothermy and fever—alters animal infection.
Our findings are posted on the open-access preprint server Biorxiv:
Palmer-Young EC, Markowitz LM, Huang W, Evans JD. High temperatures augment inhibition of parasites by a honey bee gut symbiont. bioRxiv; 2023. p. 2023.03.20.533504. doi:10.1101/2023.03.20.533504. This manuscript has been accepted by the journal Applied and Environmental Microbiology.
We also created a Youtube presentation to publicize the findings:
A heated interaction: High temperatures augment inhibition of parasites by honey bee gut symbionts
<https://www.youtube.com/watch?v=WwrZBNuP0UM>.
We are currently testing the hypotheses generated by these cell culture experiments using infection trials with worker bees. These experiments will compare the temperature dependence of symbiont colonization, trypanosomatid infection, and gut acidity in the presence vs. absence of gut microbiota. We predict that both high temperatures and the presence of symbionts will reduce infection, and that the inhibitory effects of symbionts will be strongest at temperatures at and above 33 °C—reflecting temperature-driven acceleration in symbiont growth and production of acids.
The role of the Eva Crane Trust
We are immensely grateful for and honored by the support of the Trust in these experiments, which we feel reflect the joint interests in honey bee health and pollinator ecology epitomized by Eva Crane herself. The results have allowed us to build on prior research in temperature-mediated interactions between bumble bee parasites and gut bacteria; to mentor and promote the career development of a highly motivated graduate student (Lindsey Markowitz); and to bring extramural funding to the Bee Research Laboratory that has provided this researcher with such a welcoming and productive work environment.
Dr Evan Palmer-Young
USDA Bee Research Lab., Beltsville, USA.
Ref.:ECTA_20201204_P-Young.